Magnetoelectric Multiferroics
The family of the magnetoelectric multiferroics (MF) is a very narrow set of complex materials (often perovksites) which simultaneously display two primary ferroic orders: anti-parallel magnetic orders (AFM, FiM) and ferroelectricity (FE). Unfortunately their stability is constrained by very strict structural, substitutional and symmetry laws: actually, only 13 Shubnikov point groups on 122 allow the coexistence between the two orders i.e. the breaking of space/time inversion symmetry. For this reason, it is almost impossible to find a material with such characteristics on Earth. Since 2010, at IMEM, we have been studying and preparing metastable MF by solid state chemical reaction under extreme thermodynamic condition (High Pressure and High Temperature (HP/HT), by means of Multi-anvil apparatus.
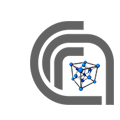
Example of antipolar space symmetry superimposed to a G-type antiferromagnetic structure
From: https://doi.org/10.1103/PhysRevB.88.014431
Highlights
-
Synthesis and characterization of complex multiferroic quadruple perovskites
We have been working on the HP/HT synthesis of the metastable quadruple perovskites and on the investigation of structural, electric and magnetic properties. These compounds are identified by the general formula AMn7O12, with A=Bi, Pb, La, Y, Tb, Nd. In particular, some of them are good candidates for type-II multiferroism. In such exotic systems, the low temperature magnetic order is directly responsible for the space inversion symmetry breaking, determining the rise of an “improper” FE state.
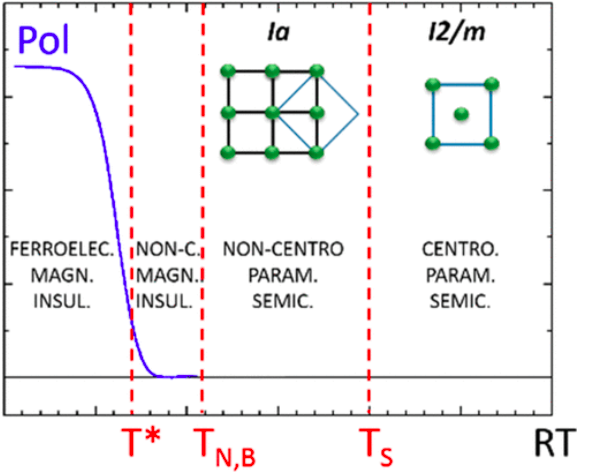
Thermal phases transition affecting both magnetic and structural characters in the metastable perovskite YMn7O12, synthesized at IMEM at 9 GPa and 1300 °C From: https://pubs.acs.org/doi/10.1021/acs.inorgchem.9b02298
-
Synthesis and characterization of new Bi-Pb based multiferroic double perovskites
We have a long tradition in the synthesis and complete characterization of the double perovskite MF systems obtained by HP/HT synthesis. The general chemical formula is A2BB’O6, where A = Bi, Pb, K, Sr, Y and B,B’ = Sc, Cr, Mn, Fe, Co, Ni, Cu, Mo, W, Re. Usually these compounds can be obtained between 3 and 6 GPa and 700-1100 °C. They are often type-I multiferroic with low correlation between the orders but higher critical temperatures.
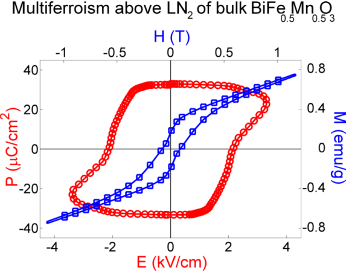
Type-I multiferroism in BiFe0.5Mn0.5O3, synthesized and firstly measured at IMEM
From: 10.1021/acs.inorgchem.6b00961
-
Synthesis and characterization of novel ferrophotovoltaic Fe-Cr-Sc-V based double perovskites
These very promising and pioneering materials are a subclass of the prev. highlight’ class we have recently started to study. The parent compound of this series is Bi2FeCrO6 which was recently discovered to be the first ferrophotovoltaic perovskite, namely both ferroelectric (and AFM) and photovoltaic material, with about 6-8% of light energy conversion efficiency in film form (Reference: https://aip.scitation.org/doi/10.1063/1.3590270).
Main Publications
- “Centrosymmetry Breaking and Ferroelectricity Driven by Short-Range Magnetic Order in the Quadruple Perovskite (YMn3)Mn4O12” - M. Verseils, F. Mezzadri, D. Delmonte, R. Cabassi, B. Baptiste, Y. Klein, G. Calestani, F. Bolzoni, E. Gilioli, A. Gauzzi, Inorg. Chem. 58(20) (2019) 14204-14211. https://doi.org/10.1021/acs.inorgchem.9b02298
- “Effect of chemical pressure induced by La3+/Y3+ substitution on the magnetic ordering of (AMn3)Mn4O12 quadruple perovskites” - M. Verseils, F. Mezzadri, D. Delmonte, B. Baptiste, Y. Klein, S. Shcheka, L. C. Chapon, T. Hansen, E. Gilioli, A. Gauzzi, Phys. Rev. Materials 1 (2017) 06440. https://doi.org/10.1103/PhysRevMaterials.1.064407
- “Crystal Structure and Ferroelectric Properties of ε-Ga2O3 Films Grown on (0001)-Sapphire” - F. Mezzadri, G. Calestani, F. Boschi, D. Delmonte, M. Bosi, R. Fornari, Inorg. Chem. 55(22) (2016) 12079-12084. https://doi.org/10.1021/acs.inorgchem.6b02244
- “Structural and magnetic characterization of the double perovskite Pb2FeMoO6” - F. Mezzadri, D. Delmonte, F. Orlandi, C. Pernechele, G. Calestani, M. Solzi, M. Lantieri, G. Spina, R. Cabassi, F. Bolzoni, M. Fittipaldi, M. Merlini, A. Migliori, P. Manuel, E. Gilioli, J. Mater. Chem. C 4 (2016) 1533-1542. https://doi.org/10.1039/C5TC03529E
- "Thermally activated magnetization reversal in bulk BiFe0.5Mn0.5O3" - D. Delmonte, F. Mezzadri, C. Pernechele, G. Calestani, G. Spina, M. Lantieri, M. Solzi, R. Cabassi, F. Bolzoni, A. Migliori, C. Ritter, E. Gilioli, Phys. Rev. B 88 (2013) 014431. https://doi.org/10.1103/PhysRevB.88.014431
Multiple-Order Systems
At IMEM, this family of perovskites is studied since the early 2000 and it represents a strong know-how of the institute. They were synthesized by means of HP/HT solid state reaction carried out in the multi-anvil press. Mixed-valence manganites with general formula (AxMn3+3)(Mn3+1+xMn4+3-x)O12 display multiple order phenomena: charge, spin and orbital order together with a variety of magnetic and structural transitions, dramatic changes of electrical conductivity and magnetoresistance effects. The physical properties vary with the relative concentration of Mn3+/Mn4+ and it can be usually changed by choosing in place of A a monovalent (Na,K), a bivalent (Ca) or a trivalent (La,Pr,Bi) ion.
Highlight
Mixed valence (Na,Ca,La) manganites with multiple order mechanisms
The reference case is constituted by the Na quadruple manganite (NaMn3+3)(Mn3+2Mn4+2)O12, where the monovalence of Na determines the necessity of having 50% occupancy of Mn3+ also in the B site. This brings to a charge ordering of Mn3+ and Mn4+ in octahedral coord., superimposed to the orbital ordering (since all the Mn4+ owns isospheric shell while the Mn3+ is a Jahn Teller active ion). Consequently, two independent magnetic lattices are created: the strongest is weak a FM constituted by the B-site Mn, while the weakest is an AFM ordering due to the A-site Mn.
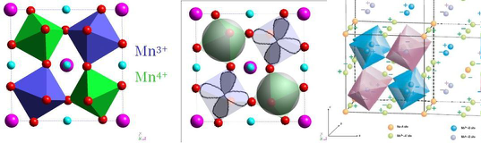 (NaMn3+3)(Mn3+2Mn4+2)O12 charge, orbital and spin ordered cell
(NaMn3+3)(Mn3+2Mn4+2)O12 charge, orbital and spin ordered cell
From: 10.1038/nmat1038
Main Publications
- “Evolution of Magneto-Orbital order Upon B-Site Electron Doping in Na1−xCaxMn7O12 Quadruple Perovskite Manganites” - R.D. Johnson, F. Mezzadri, P. Manuel, D.D. Khalyavin, E Gilioli, P.G. Radaelli, Phys. Rev. Lett. 120(25) (2018) 257202. https://doi.org/10.1103/PhysRevLett.120.257202
- “Polymorphism and Multiferroicity in Bi1–x/3(MnIII3)(MnIII4–x MnIV x)O12” - F. Mezzadri, M. Buzzi, C. Pernechele, G. Calestani, M. Solzi, A. Migliori, E. Gilioli, Chem. Mat. 23(16) (2011) 3628-3635. https://doi.org/10.1021/cm200879p
- “Magnetic structure of the high-density single-valent e g Jahn-Teller system LaMn7O12” - A. Prodi, E. Gilioli, R. Cabassi, F. Bolzoni, F. Licci, Q. Huang, J. W. Lynn, M. Affronte, A. Gauzzi, M. Marezio, Phys. Rev. B 79 2009, 085105. https://doi.org/10.1103/PhysRevB.79.085105
- “High-pressure synthesis and characterization of PrMn7O12 polymorphs” - F. Mezzadri, M. Calicchio, E. Gilioli, R. Cabassi, F. Bolzoni, G. Calestani, F. Bissoli, Phys. Rev. B 79 (2009) 014420. https://doi.org/10.1103/PhysRevB.79.014420
- “Charge, orbital and spin ordering phenomena in the mixed valence manganite (NaMn3+3)(Mn3+2Mn4+2)O12” - A. Prodi, E. Gilioli, A. Gauzzi, F. Licci, M. Marezio, F. Bolzoni, Q. Huang, A. Santoro, J. W. Lynn, Nat. Mat. 3 (2004) 48-52. https://doi.org/10.1038/nmat1038